Abstract
Background
Autoimmune diseases and infections have been reported to increase the risk of acute myeloid leukemia (AML). However, most studies lacked information on prior hematological diseases and cytotoxic therapy and thus, such factors and not inflammation may explain prior findings. To test the hypothesis that inflammation has no or little impact on leukemogenesis, we investigated whether the risk of AML was decreased in individuals using anti-inflammatory drugs.
Methods
We conducted a nationwide case-control study, using the Danish National Acute Leukemia Registry to all AML patients diagnosed from 2000 to 2013, and selected 10 population controls per case matched on sex and age. To avoid recall bias, we used the Danish National Health Service Prescription Database to identify all prescriptions redeemed from 1995 to 2013 and before the AML diagnosis/index date. We used conditional logistic regression analysis to compute odds ratios (ORs) associating NSAID. To avoid reverse causation we ignored prescription 3 years before diagnosis/index date. Results were given for overall use, by NSAID subtype, by number of prescriptions both overall and for de novo AML and AML related to previous hematological disease (secondary AML) or therapy-related AML (previous cytotoxic therapy). Results were further stratified by sex and age (<60 years, ≥60 years).
Results were given with 95% confidence intervals (CIs).
Results
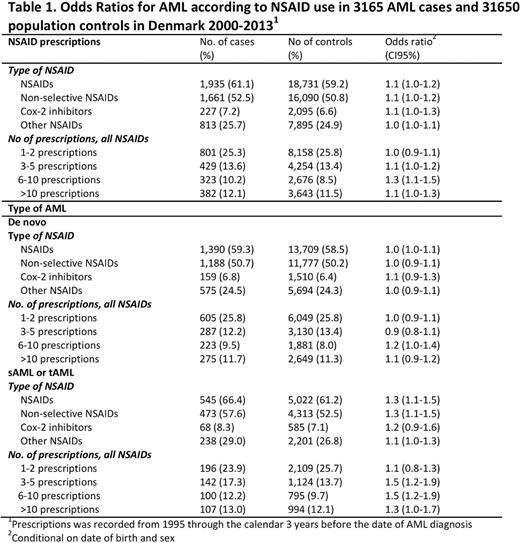
The final study population included 3165 AML cases and 31,650 sex- and age-matched population controls. The median age was 66.8 years (range 15-99). In total, 68% of cases and 66% of controls redeemed NSAID prescriptions before the AML diagnosis or the index date.
The associations between NSAID use and risk of AML overall and by AML type are shown in Table 1. Overall, any-use of NSAID was associated with an increased risk of AML (OR 1.1 (CI=1.0-1.2)). Similar effect was seen across NSAID subtypes, though results were less precise.
Long-term use did not reduce the risk of AML, when comparing the risk of AML between patients with different numbers of prescriptions (1-2 prescriptions OR 1.0 (CI=0.9-1.1) vs. >10 prescriptions 1.1 (CI=0.9-1.2)).
Repeating analysis for de novo AML and sAML/tAML patients, separately, the increased risk of AML in NSAID users across NSAID subtype and numbers of prescriptions could be contributed entirely to sAML/tAML patients (OR 1.3 (CI=1.1-1.5)), whereas no association was found between NSAID consumption and risk of de novo AML (OR 1.0 (CI=1.0-1.1)).
Stratification by acute promyelocytic leukemia (APL)/non-APL, sex or age did not change the interpretation of results (results not shown).
Conclusion
In this large population-based study we did not find NSAID to reduce the risk of AML. In contrast, a higher risk of sAML/tAML was seen in NSAID users, likely reflecting higher use of NSAIDs in in patients with an a priori increased risk of AML. Neither age, sex, type of NSAID, nor number of prescriptions influenced the results. These findings support the hypothesis that inflammation is an unlikely causal factor for AML.
Østgård: The Danish Cancer Society: Research Funding; Pfizer: Other: Travel expences. Nørgaard: Program for Clinical Research Infrastructure (PROCRIN): Research Funding. Pedersen: Program for Clinical Research Infrastructure (PROCRIN): Research Funding. Østgård: AbbVie: Consultancy; Pfizer: Consultancy; Bristol-Myers Squibb: Consultancy; Lilly: Consultancy. Severinsen: Novartis: Research Funding; Celgene: Research Funding; ARIAD: Research Funding. Schöllkopf: Karyopharm Therapeutics: Research Funding; Teva: Other: Travel expences; Pfizer: Other: Travel expences. Jensen: The Dagmar Marshall Foundation: Research Funding; The Danish Acute Leukemia Group: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal